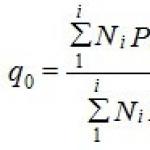The process of intergranular corrosion of solid metals in a liquid metal medium has not been specifically studied. The following are some of the likely mechanisms of this process, the existence of which is confirmed by indirect experimental observations.
1. One of the reasons for intergranular corrosion is a higher potential energy level of atoms located in intergranular zones, compared with atoms inside crystallites. Consequently, the activation energy of dissolution for these atoms is less than for the rest. Accordingly, the probability of their release into the solution ωt increases. It was previously shown that the dissolution rate constant for the process controlled by the first stage is α \u003d ωtρ "/ n∞. Thus, an increase in the probability of release into the liquid metal solution for atoms of intergranular zones means that the dissolution rate of these sections of the metal structure is higher than the rate As a result, the corrosion front under the conditions of isothermal and non-isothermal dissolution will deepen along the grain boundaries of the metal, that is, intergranular corrosion will occur. . Dissolution of the grain boundary is so large that the matrix is \u200b\u200bseparated from whole grains example heavy intergranular fracture is corroded nickel in the liquid lithium at 1000 ° C, the metal microstructure is characteristic for this case is shown in Figure 42..
Care must be taken to ensure that contaminated pollution does not spread over the surface over a large area. Against paints, there are special alkaline and solvent based cleaning products. Chloride-containing products, in particular products containing hydrochloric acid, bleaches and cleaning agents for silver should not be used.
We provide information materials presented here for free to visitors to our website as a service. The contents of our pages have been created with great care. However, for the correctness, completeness and relevance of liability and warranty is not allowed.

Let us estimate the depth of intergranular corrosion of a metal caused by the difference in the rates of dissolution of atoms from the grain body and intergranular zones. For isothermal dissolution, in this case, the number of atoms passing into the liquid metal solution per unit time is determined by a dependence similar to equation (1):
where the index "z" means that the corresponding characteristic refers to dissolution from the grain surface, and the index "g" refers to dissolution from the intergranular zone. Considering, as before, that N-nVж, we obtain the differential equation

The content and structure of information materials are protected by copyright. All stainless steels contain enough chromium to give them stainless steel properties. Many stainless alloys also contain nickel to further enhance their corrosion resistance. These alloys are added to the steel in a state of melting to make it "stainless in all its mass." For this reason, stainless steels need neither veneering, nor painting, nor any other surface treatment to improve their corrosion resistance.
There is nothing in stainless steel that can be cleaned, worn, jump or tear. If it is not burned, oxidation continues until the steel is completely corroded. Stainless steels also oxidize, but instead of the usual oxide, a weak film of very dense chromium oxide forms on the surface, forming a corrosion-resistant coating. If this chromium oxide film covering stainless steels is removed, it immediately forms when chromium combines with oxygen in the surrounding atmosphere.
Solving equation (95) and using the initial condition: t \u003d 0, n \u003d 0 and the saturation ratio dn / dt \u003d 0, we find the kinetic equation for isothermal dissolution taking into account the influence of intergranular zones in the following form:
To determine the depth of general (lz) and intergranular (lg) corrosion, we write the differential equation
where ρз and ρг are the bulk density of crystallites and intergranular zones, respectively. We obtain a solution to this equation using equality (96) and the initial condition t \u003d 0, lz \u003d 0, lg \u003d 0:
In order to obtain the ratio of the depth of intergranular to general corrosion, we write the following approximate expression for the latter:
where the symbol a denotes, as before, the constant of the dissolution rate equal to ωt * ρ "/ n∞, and nг∞ is that part of the solution concentration that is achieved by dissolving the metal of the intergranular zones. Subtracting (99) from (98), we find :
We divide equation (100) by (99), neglecting the second term on the right-hand side of equation (99), which is obviously much smaller than the first. Then we obtain the desired ratio in the form
From equation (101) it follows that intergranular corrosion increases with time.
Of greatest interest is the maximum value of the ratio lg / lz, which is achieved by the end of the dissolution process. From (101) we find that when the solution is saturated, i.e., as t → ∞, this ratio is
The value (lg / lz) max can be estimated as follows. In a first approximation, we can assume that nг∞ / n∞≈αgSg / αзSз; further, taking into account that α \u003d ω * ρ "/ n∞ and ρ" \u003d α * ρ, where α is the interatomic distance, we obtain (lg / lz) max≈ωg / ωz. The probability of the transition of a solution of atoms from the surface of the grain is expressed by the dependence ωz \u003d v exp (-Qp / RT). Due to the fact that the activation energy of dissolution from intergranular zones is less than that from the body of crystallites by the amount of their excess energy ΔQg, the probability of transition of atoms from these sites to the solution will be ωg \u003d v * exp [- (Qр-ΔQg) / RT] Using these expressions, we obtain (lg / lz) max ≈ exp (ΔQg / RT).
We will numerically estimate the ratio of the depth of intergranular corrosion to the total for γ-iron. The average value of the free energy of the grain boundaries in him, according to the work, is 8040 cal / g * atom. Given this, we find that at a temperature of 800 ° C the ratio (lg / lz) max is approximately 40. Therefore, the depth of intergranular corrosion in iron can be almost 40 times the depth of general corrosion. It should be noted, however, that with a significant deepening of the corrosion front along grain boundaries, the dissolution process will be hindered by the diffusion of dissolved atoms through the liquid metal in the formed narrow and long channel, which can be considered as an increase in the thickness of the boundary film in this region. Such a process, of course, will limit the depth of intergranular corrosion.
The ratio of the depth of intergranular corrosion to the depth of general corrosion under thermal mass transfer can be obtained using the equation of mass transfer in the form
where Δt is the transit time of the hot zone by the flow of liquid metal. Since the weight of the metal transferred to the cold zone over time t is ΔP \u003d S * Rpm * t, where S is the surface from which dissolution occurs in the hot zone, then on the basis of equality (103) we obtain
We turn now to Fig. 43, which shows the kinetics of dissolution of the metal of crystallites and intergranular zones. It can be seen from the schemes that the initial (ϗ * nн∞ + nк) and final (nв) concentrations of the solution in the hot zone are the same for crystallites and intercrystalline zones, but the Δt value is different for them, which is associated with a different value of αg and αz . Bearing in mind that the area of \u200b\u200bthese structural sections also differs, that ΔP \u003d ρSl, and using the ratio of beca of the metal dissolved from intercrystallite zones and from crystallites, we find
Since ρз≥ρg and Δtз≥Δtg, lg / lз≥1, i.e., intergranular corrosion also occurs under conditions of thermal mass transfer. If nv is significantly less than nv∞, then we can use the approximate equality Δtg / Δtz \u003d αz / αg (see Fig. 43). Based on the last relation, equation (105) takes the form lg / lz≈ρz / ρg * αg / αz. Therefore, the intensity of intergranular corrosion in this case is determined by the ratio of the dissolution rate constants of the metal of the intergranular zones and crystallites. It is interesting to note that the rate of destruction of the metal along the boundaries of crystallites during thermal mass transfer is equal to the maximum value of the ratio lg / lz during isothermal dissolution. Therefore, it is possible to use the previously made assessment of this ratio, from which it follows that the depth of intergranular corrosion, for example, iron at 800 ° C, can be approximately 40 times the depth of general corrosion. At the same time, it must be emphasized that if the intensity (lg / lz) of intergranular corrosion changes little over time, then the difference between the depth of intergranular and general corrosion continuously increases. So, the depth of general corrosion under conditions of mass transfer is determined by the equation
and the intergranular corrosion depth for the case considered above is equal to
whence follows
Thus, in this case, the difference between the depth of intergranular and general corrosion increases with time according to a linear law. However, such a development of intergranular corrosion cannot be unlimited. As already indicated, the maximum depth of intergranular corrosion is determined by the moment of transition to the control of the dissolution process in the channels formed by neighboring crystallites by a diffusion mechanism.
The destruction of grain boundaries due to the increased energy of the atoms located here can occur during dissolution, as well as in isothermal conditions when the solution reaches saturation. In the latter case, corrosion will be carried out by energy transfer of the mass. This sometimes explains the effect of liquid metals on solid metals during isothermal tests of long duration, although the solution has long reached equilibrium concentration.
It should be noted that the energy transfer of mass is local in nature and covers only small areas of the surface. This feature of it is explained by the fact that the driving force of transfer is the energy gradient dU / dx, where x is the distance along the surface. metal. The process of energy transfer of mass is a set of processes of dissolution, diffusion in the surface film of liquid metal and crystallization. The diffusion rate in this case is determined by an equation similar to (78):
where D is the diffusion coefficient in liquid metal; S is the surface area through which diffusion occurs; f is the coefficient of proportionality. Obviously, at a sufficiently large distance between regions with different atomic energies, the energy gradient will be small, and the diffusion rate will be negligible. As a result, the transfer process between these sections will not practically occur.
2. Intergranular corrosion of alloys may be associated with selective corrosion. This effect should be observed in two cases. If the readily soluble element is horophilic, then, naturally, its preferential dissolution will to a greater extent cause destruction of the intergranular zones than the crystallites themselves, where the initial concentration of this element is much lower. An example of such an effect is apparently the selective dissolution of nickel from austenitic steels. It is known that steels of this class are usually subjected to intergranular corrosion in liquid metals and this effect is especially pronounced when testing steels in lead and bismuth. If we take into account that nickel is a horophilic element in iron alloys, then this effect finds an explanation.

The second case of intergranular corrosion of selectively dissolving alloys is possible with a uniform distribution of an easily soluble element in the matrix. The condition providing local destruction of the alloy along the grain boundaries, in this case, is a higher diffusion rate of the readily soluble element along the grain boundaries than by their volume. The intergranular corrosion of chromium steels observed in liquid bismuth is apparently associated with the predominant boundary diffusion of chromium, since, according to the data, it is not horophilic in iron-based alloys. In some alloys, the readily soluble element can be horophilic and have a higher coefficient of boundary diffusion, which should lead to a significant increase in the intergranular corrosion of the alloy in a liquid metal.
In the case of chemical interaction of the liquid metal with the component or components of the alloy, intense intergranular fracture can also be observed due to the above reasons.
3. The destruction of solid metals along grain boundaries in a liquid metal medium can occur at a certain ratio of free surface energy of the boundary of two grains and free energy of the solid-liquid metal interface.
Consider the equilibrium condition of surface tension at the meeting point of the boundary of two grains with a liquid metal (Fig. 44). We denote by γmt the surface tension of the boundary of two grains, and γm by the surface tension of the boundary of each grain with liquid metal (we assume that γm does not depend on the orientation of the grain). Further, let θ be the dihedral angle between the contact surfaces of two neighboring grains with a liquid metal medium. Then the equilibrium condition, in accordance with the scheme in Fig. 44 will
Thus, depending on the ratio of surface tension values, the surface relief of the solid metal at the exit point of the grain boundary will be different. If the equilibrium condition is met by a small acute angle, then in this case intergranular corrosion should be observed. Moreover, with a decrease in the dihedral angle, intergranular corrosion will intensify. At θ \u003d 0, the medium will penetrate deep into the solid metal along the grain boundaries and divide into separate grains. In the other extreme case, at θ \u003d 180 °, there will be no intergranular corrosion. The angle interval 90 ° ≤θ≤180 ° can be considered as the case of the formation of small grooves along the grain boundaries that are found on the polished surface of a solid metal after a short dissolution in a liquid metal. Obviously, the lower boundary (90 °) is conditional, since even at lower dihedral angles, intergranular corrosion is small. Apparently, 0 should be considered a particularly dangerous range of values \u200b\u200bof 0. In view of the extreme complexity of the experimental determination of the free surface energy of solids and the energy of the solid-liquid metal interface, these quantities are known only for very few materials. There are also no sufficiently reliable methods for their theoretical calculation. Therefore, the above considerations cannot be applied to combinations of metals of interest in our case. As an illustration of the described effect, we indicate the intergranular penetration of bismuth into copper and the absence of damage to the grain boundaries when copper is immersed in lead. Considering the almost complete wetting of copper by bismuth (the edge angle is close to zero) and poor wetting by lead, the difference in the effect of these liquid metals becomes clear. By adding zinc and tin to bismuth, which increase the energy of the copper - bismuth interface, the intergranular corrosion of copper at 600 ° C was eliminated.
It should be noted that in the case of simultaneous occurrence of various types of corrosion, the values \u200b\u200bof the surface energies of the boundary of two grains and the interphase boundary can change significantly over time, which will cause a corresponding change in the dihedral angle. The energy of the boundary of two grains can change as a result of selective corrosion or boundary diffusion of a liquid metal. Interfacial energy can change its value due to the formation of a solid solution or intermetallic compound on the metal surface, as well as due to a change in the composition of the liquid metal medium.
Intergranular corrosion, due to a certain ratio of surface energies, can occur both in the process of dissolution and after saturation of the solution by energy transfer of the mass.
4. Intensive destruction of solid metals along grain boundaries is observed when there are impurities in the liquid metal. The most characteristic example is the intergranular corrosion of materials in liquid sodium, which contains a significant admixture of oxygen. So, stainless chromium and chromium-nickel steels and nickel-base alloys undergo intergranular corrosion in sodium with an admixture of 0.5 wt.% Oxygen at 700 ° C.
The reason for this effect of oxygen is the chemical interaction of oxygen or sodium oxide ions with alloy components lying in intergranular zones. Due to the small volumes in which this interaction takes place, and the small amount of reaction products, the processes of intergranular corrosion in liquid metals with impurities have not yet been studied.
5. Intergranular corrosion can also be observed during the interaction of alkali metals with oxides, sulfides, phosphides and carbides located in solid metals mainly along grain boundaries. Such processes will be discussed in the next chapter.
I. PURPOSE OF WORK
The use of stainless steel will depend on the oxidizing properties of the environment. If strong oxidizing conditions prevail, stainless steels surpass the most noble metals and alloys. However, in the same stainless steel family, corrosion resistance varies significantly from one type to another.
The use of chromium steels for industrial purposes is mainly due to oxidation resistance conditions. Chrome steel with 12% will form surface rust after several weeks of exposure to an industrial atmosphere. Once formed, the film acts as a barrier against the most pronounced corrosion, but if it is necessary to take into account the appearance of the metal, type 410 and type 405 may be undesirable. Type 430 with a chromium content of 17% requires several months to form a surface oxide film, while type 442 with more than 20% chromium becomes passive in the atmosphere without the formation of a visible oxide film.
Get familiar with intergranular corrosion detection methods
steel and ways to deal with it.
II. THEORETICAL SUBSTANTIATION
In stainless steels, carbon can be present in carbides, which in the electrolyte will be more electropositive than, for example, ferrite. Therefore, there is electrochemical heterogeneity - one of the necessary prerequisites for the occurrence of electrochemical corrosion.
In atmospheres containing salty air or fumes in chemical plants, the addition of molybdenum increases the corrosion resistance, as is the case with type 316. If we briefly dwell on the latest developments in the field of stainless steel finishes and fittings used in automobiles, what we only what they said will be more clearly illustrated. American automakers used Type 430 for body trim and trim, and Type 301 for wheel covers and trim, which are hard to shape.
However, with the increase in the use of aggressive and abrasive salts to accelerate the thawing of streets and roads in the winter, malfunctions of type 430 also increased. On the contrary, type 301 for finishing successfully passed corrosion attacks. This procedure prevents the migration of chromium from the surface.
In the consumption of stainless chromium-nickel steels the maximum
the specific gravity (about 80%) is still a universal alloy of the austenitic class X18H9 type. These alloys have medium strength characteristics ( in 700 MPa), high ductility ( 40%), good weldability, high corrosion resistance in many aggressive environments: in organic (acetic, picric) and nitric acids, sea water, moist air , solutions of many salts and alkalis.
Type 434 was also developed, containing 17% chromium and 1% molybdenum to obtain higher resistance to the corrosive salts used to deflate routes, while at the same time meeting the requirements of more complex production for many body parts. Vibrant annealing also made the use of type 301 more advanced for body parts curved with cylinders.
There are five risks that threaten the success of using stainless steels. These are: intergranular corrosion, galvanic corrosion, contact corrosion, piercing or pin corrosion, and fatigue corrosion. Many failures can be avoided simply by realizing the risks involved and taking appropriate steps to address them.
The high corrosion resistance of austenitic chromium-nickel steels is due to easy passivation, in which chromium plays the main role. The state diagram of iron-chromium is shown in Fig. 4.1.
Chrome narrows the область region, which closes at 12% chromium and 1000 ° C. Carbon, on the contrary, expands the region and binds chromium into cubic Cr 23 C 6 and trigonal Cr 7 C 3 carbides, depleting the solid solution with chromium (1% carbon binds about 10 ... 12% chromium).
Improper heat treatment of stainless steel can lead to crosslinking of carbide in steels with a carbon content of more than 0.03% or without the addition of titanium or columbia. A metal containing such a reticulum is susceptible to intergranular corrosion, which can lead to destruction under conditions of high corrosion activity and shorten the life of many relatively lightweight services. Conventional welding processes introduce susceptibility to carbide precipitation into the metal. This steel is subject to intergranular corrosion; it does not necessarily mean that it will be attacked by it.
Chromium promotes the transition of iron to a passive state, while obeying the rule of stability boundaries ( tamman rule n / 8).
According to this rule, the corrosion resistance of the solid solution is not directly dependent on the composition of the alloy, but varies with  strokes. A sharp change in corrosion resistance occurs when the concentration of chromium or another alloying element reaches 1/8 atomic fraction or a multiple of this number, i.e. 2/8, 3/8, 4/8, etc. The position of the stability boundary (the value of n depends on the nature of metals and the degree of aggressiveness of the medium). For example, the Fe-Cr-C alloy in 50% HNO 3 at 90 ° C has three (n \u003d 1, 2, and 3) stability boundaries (Fig. 4.2), the Fe-Cr alloy in FeSO 4 solution has one (Fig. .4.3).
strokes. A sharp change in corrosion resistance occurs when the concentration of chromium or another alloying element reaches 1/8 atomic fraction or a multiple of this number, i.e. 2/8, 3/8, 4/8, etc. The position of the stability boundary (the value of n depends on the nature of metals and the degree of aggressiveness of the medium). For example, the Fe-Cr-C alloy in 50% HNO 3 at 90 ° C has three (n \u003d 1, 2, and 3) stability boundaries (Fig. 4.2), the Fe-Cr alloy in FeSO 4 solution has one (Fig. .4.3).
In the process of servicing, the result may be satisfactory. But the possibility of intergranular corrosion should be considered if it is not excluded in accordance with previous experience. Heat treatment located in the immediate vicinity of the weld does not give satisfactory results. For effective annealing, the entire workpiece must be heated and quickly cooled.
The danger inherent in the deposition of chromium carbide has become so famous and so easy to avoid that several failures have occurred due to this reason. Galvanic corrosion has a localized effect that can occur when a compound between two dissimilar metals is immersed in a solution that can act as an electrolyte. In a corrosive environment, two different metals form short-circuited electrodes and represent an electrochemical cell. This leads to dissolution of the anode electrode, while the electrode remains unchanged.
 Rule n / 8 is of great practical importance, as it allows rationally alloying a solid solution in order to increase corrosion resistance. So, a sharp increase in corrosion resistance (electrode potential), shown in Fig. 4.4, corresponds to the content in the solid solution of 1/8 atomic fraction of chromium, which is 12.5% \u200b\u200b(atomic) or 11.7% (by weight). The higher chromium content practically does not increase the corrosion resistance of iron (Fig. 4.1, 4.2).
Rule n / 8 is of great practical importance, as it allows rationally alloying a solid solution in order to increase corrosion resistance. So, a sharp increase in corrosion resistance (electrode potential), shown in Fig. 4.4, corresponds to the content in the solid solution of 1/8 atomic fraction of chromium, which is 12.5% \u200b\u200b(atomic) or 11.7% (by weight). The higher chromium content practically does not increase the corrosion resistance of iron (Fig. 4.1, 4.2).
The potential will vary depending on the position occupied by metals and alloys in the table of galvanic series that accompanies it. The use of various metals in a corrosive solution does not mean that galvanic corrosion is inevitable. Factors affecting galvanic corrosion include.
The metal, which occupies the highest position in the series, constitutes the cathode. Another metal is the anode, and because of it the one that is attacked by the action of the cell. The potential increases, and the positions occupied by each metal in the series differ from each other. Thus, in an oxidizing solution, passive stainless steels usually constitute the cathode, while other metals will be attacked. When the solution decreases, stainless steel becomes active, and metals such as copper and bronze form a cathode and accelerate corrosion of stainless steel.
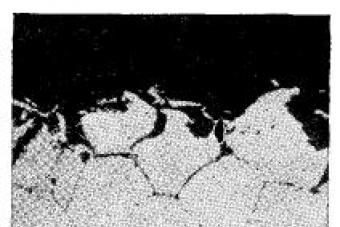
FROM 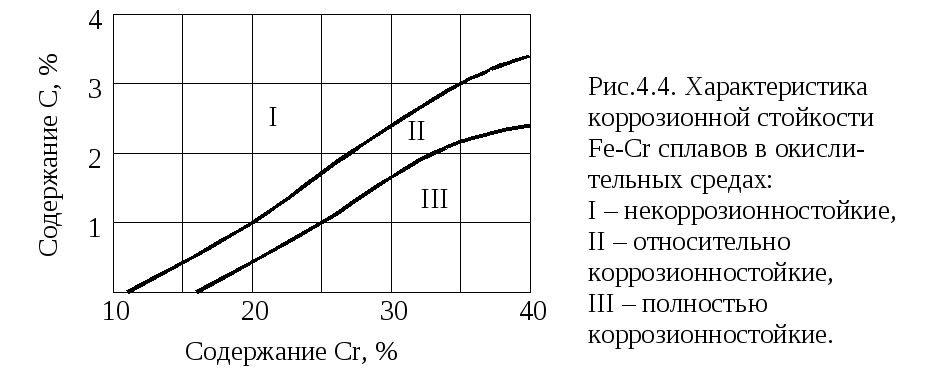 a significant drawback of X18H9 austenitic stainless steels is their tendency to intergranular corrosion under certain conditions. Intergranular corrosion is one of the most dangerous types of corrosion damage, since often, without changing the appearance of the metal structure, it leads to a sharp decrease in strength and ductility.
a significant drawback of X18H9 austenitic stainless steels is their tendency to intergranular corrosion under certain conditions. Intergranular corrosion is one of the most dangerous types of corrosion damage, since often, without changing the appearance of the metal structure, it leads to a sharp decrease in strength and ductility.
Steel and cast iron foundries occupy lower positions in the galvanic series than those occupied by active stainless steel, which will be attacked if a cell and stainless steel form between them, the same if they are immersed in an oxidizing solution, which is in the gearbox.
The evolution of hydrogen ions can passively change the active surface of stainless steel, thereby accelerating the corrosion of the anode. A small anode with a large cathode creates a high-density current and accelerates corrosion in the anode. Small areas of less noble metal should be avoided. Aluminum fasteners for stainless steel will not be used. On the other hand, the use of aluminum stainless steel fasteners gives satisfactory results.
Intergranular corrosion of austenitic chromium-nickel steels is associated with low stability of grain boundaries after slow cooling of steel in the temperature range 450 ... 850 ° C, which occurs mainly during welding.
To explain causes of intergranular corrosion There are several theories, of which the theory of depletion of grain boundaries by chromium is the most common and experimentally proven. According to this theory, when heated in the temperature range 450 ... 850 ° C, chromium-rich carbides Cr 23 C 6 or (Cr, Fe) 23 C 6 are precipitated along the grain boundaries. Almost all alloy carbon is involved in the formation of these carbides, and chromium is only located in areas adjacent to grain boundaries, which is explained by the high diffusion rate of carbon compared to the diffusion rate of chromium at the above temperatures. Due to the formation of carbides, the grain boundary regions are depleted in chromium, and when the chromium content is less than 1/8 of the atomic fraction (less than 11.7% by mass), these regions lose their passive state (see Fig. 4.4).
Corrosion is often attributed to galvanic action when its true cause is actually related to abnormal operating conditions. So, for example, using hydrochloric acid to replace conventional cleaning material can destroy a passive stainless steel film. In this case, a galvanic cell may form, which will begin to function as soon as this part comes into operation. Redesigning and building a piece made entirely of stainless steel can be very expensive, and projecting a new product can be difficult.
The tendency of stainless steels to intergranular corrosion is determined on the samples. Testing of rolled products, forgings, pipes, welds, wire, casting is envisaged. Fat-free and dried samples with a surface roughness class of at least 7 are tested for intergranular corrosion according to one of the following in table. 4.1 methods.
Thus, when it turns out that galvanic action is the only cause of a malfunction in the unit, which is clearly a good design, a thorough check must be made to ensure that all operating conditions are normal. The third risk is contact corrosion. A small particle of carbon steel, a scale of oxide, copper, or other foreign matter embedded in stainless steel, may be sufficient to break passivity at the point of contact. The attack begins with the formation of a galvanic cell with a particle of foreign material in the form of an anode.
At the end of the AM test, the samples are removed from the flask or tank, washed, dried and bent at an angle of 90 °. The presence of transverse cracks on the surface of a bent sample indicates the tendency of steel to intergranular corrosion. When tested according to method B, such evidence is the presence of a continuous grid at the sites of anode etching. When tested by method D, steel is considered to be prone to intergranular corrosion if the corrosion rate of steel after any cycle exceeds 2 mm / year or if knife corrosion is observed on welded specimens, having the form of a notch with a knife in the fusion zone of welded joints (Fig. 4.2, 4.3 )
During an electrochemical action that dissolves contaminated hydrogen ions are released, which leads to the fact that stainless steel becomes active at the point of contact. Grinding can continue after the removal of foreign matter due to the fact that between the small surface of the anode surface and the vast surrounding area of \u200b\u200bthe cataract, an active-passive cell is formed. When commissioning stainless sections, they must be cleaned of scales of oxide, oil, small metal particles from tools, dies and rows, as well as from any foreign materials.
The development of intergranular corrosion can be observed in several ways:
periodically remove samples from the solution and measure their electrical resistance: an increase in electrical resistance indicates the development of intergranular corrosion;
periodically remove samples from the solution and throwing them onto a solid plate (tile, glass, etc.), by sound, judge the development of intergranular corrosion: with deep intergranular corrosion, the sample (if it is not covered with copper deposits) loses a metallic ring;
Contact corrosion can begin after a long service time if the cleaning methods used are not meticulous. Oxidation and dirt in steam pipes, carbon steel impregnated tools, and even dirty transport equipment can lead to contact of corrosive substances up to stainless steel containers during the cleaning period. Clean and smooth surfaces, as well as the absence of scratches and cracks, reduce the risk of contact corrosion.
The design engineer can be careful in any galvanic attack, but in turn, the personnel responsible for the production, operation and maintenance of stainless steel equipment must prevent contact corrosion.
expose the samples to a cold bend of 180 °: cracks form in the sample with intergranular corrosion in the places of bend;
examine the microsection under a microscope: the grain boundaries of steel with intergranular corrosion look wide and dark.
Struggle against intergranular corrosion lead by preventing the formation of chromium carbides at the grain boundaries:
Pitting or pin corrosion. Chloride-containing solutions can be pulverized, and can be developed in pitting galvanic cells. Damage caused by this blow is also called pin piercing caused by corrosion. Chloric acids, such as iron chloride and sodium chloride, are especially dangerous, but any chloride in appreciable concentration may be a possible cause of the disturbance. As a rule, stainless steel failures in an environment that is supposedly safe from corrosion are explained by the presence of a chloride ion in a higher concentration than predicted.
Carbon reduction;
Hardening;
Long-term heating at 860 ... 880 ° C;
Additional alloying.
Table 4.1. Intergranular corrosion test methods
Carbon. as its content decreases, the tendency of chromium-nickel steels to intergranular corrosion decreases. With a carbon content of less than 0.015%, these steels are practically not prone to this type of corrosion.
Quenching. As a result of quenching in water from temperatures of 1050 ... 1100 ° C, carbon and chromium are fixed in a solid solution, which is favorable in terms of corrosion.
Long (more than two hours) heatingat temperatures of 860 ... 880 ° C. With such heating, carbides cease to be released and their coagulation proceeds, and therefore the continuity of the carbide network and chromium depleted regions along grain boundaries is disrupted. Moreover, chrome, due to prolonged exposure, manages to diffuse into depleted areas, which leads to equalization of its concentration and increase the passivation of steel. When heated to 860 ... 880 ° C, the internal stresses arising from the formation of carbides are completely removed, and this also contributes to an increase in corrosion resistance.
Additional alloying elements that bind carbon to more difficultly soluble carbides compared to chromium, prevents the occurrence of intergranular corrosion. Such alloying elements are Ti, Nb, Ta. For complete binding to carbides, there must be some excess of these elements with respect to the stoichiometric composition (TiC and others). However, the addition of alloying elements in large quantities can lead to the formation of a ferrite component, which does not reduce, but even accelerates the development of intergranular corrosion.
Microstructures of Cr-Ni steels of austenitic class after quenching without provoking heating and after quenching, followed by prolonged heating at elevated temperatures.

intergranular
healthy microstructure (not affected by MCC)
Table 4.2. Determination by sound the presence or absence of intergranular corrosion in steel samples 08Kh18N10T.
Conclusions on the effect of Ti on the tendency of Cr-Ni steels of the austenitic class to intergranular corrosion.
Ti is an alloying element that binds carbon to more difficultly soluble carbides compared to chromium, and prevents the occurrence of intergranular corrosion. For complete binding to carbides, there must be some excess amount of this element with respect to the stoichiometric composition (TiC and others). However, the addition of alloying elements in large quantities can lead to the formation of a ferrite component, which does not reduce, but even accelerates the development of intergranular corrosion.