October 25, 2013
Synthesis of acids
In such a science as chemistry, special attention is paid to the synthesis of those compounds that simply cannot be found in nature. Using the unique properties of such compounds, many unique problems can be solved.
When creating unique synthesized acids, the storage of these compounds and their stability can become a major problem. There are acids that dissolve glassware or those that have a lifetime of milliseconds, which will make it impossible to make observations and use chemical properties, so the task of creating stable connections is the most important.
Acid theories
There are two theories of acids in the world. The first is the Brønsted-Lowry theory, which promotes the protonic version of acids. Such compounds are capable of donating a proton during the reaction. The proton in such compounds is bonded to a base, which has the opposite charge. And the more protons (hydrogen ions) an acid can give away, the stronger it is considered. The proton, in order to balance its charge, has a very high activity and tries to capture an electron from other compounds into its orbit. This explains the high chemical activity of known mineral acids.

The second theory, which is called the Lewis theory, states that the acidic properties are also exhibited by those compounds that form during the reaction covalent bonds. Pairs of electrons of the reacting substances combine and become common to both atoms. According to this theory, not only protons have acidic properties, but also compounds that have activity in creating electron pairs. Thus, the Lewis theory significantly expanded the Bronsted-Lowry theory and many more compounds known to science were included in the class of acids.
Modern chemical synthesis has reached unprecedented heights. We owe him the appearance of kapron, nylon, dacron, lavsan, spandex, lycra. Modeling the desired properties of a synthesized substance on a computer, and then creating it, has no longer become a fantasy. Scientists and chemists are like children who assemble spatial figures from a constructor, and then study what they have created. Chemical synthesis allows you to create substances that cannot exist in nature, and therefore with unknown, interesting and useful properties.
Carboranoic acid
A group of scientists from the University of California, together with scientists from the Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences, set themselves the task of synthesizing a strong acid that would not yet be aggressive to surrounding materials. This, at first glance, an impossible task, was solved. The created compound, according to scientists, is a million times stronger than high concentration sulfuric acid and is inert to glass vessels. Any compound whose acidity exceeds that of 100% sulfuric acid is already commonly referred to as superacids. Then what can you call a compound that is a million times stronger?

The studies carried out allow us to assert that carborane acid (namely, this is how it was given the name) is the strongest acid of the currently studied.
This compound has the chemical formula H(CHB11Cl11) gives the solution much more hydrogen ions (protons) than all the others, and the remaining base has amazing inertness. This group contains 11 boron atoms, 11 chlorine atoms and a carbon atom - which are linked in a spatial structure in the form of an icosahedron. It is known that figures with the structure of the Platonic solids (namely, this is the icosahedron) have very high strength. And it is precisely such an effective spatial organization of the base that allows it to exhibit chemical inertness.
Practical value
Carborane acid, in addition to the scientific value of its discovery and synthesis, can also be of considerable practical value. With the help of this unique compound, the synthesis of organic "acidic" molecules is planned, which are formed in the human body for a very long time. a short time during the digestion of food and therefore little studied. Such a stable structure of the base gives scientists the right to assume the use of this acid in the pharmaceutical and chemical industries as a catalyst.

It does not give rest to scientists chemists all over the world to create a combination of hydrogen with inert gases, which are always "reluctantly" combined with other elements of the Periodic Table. Currently, only compounds of xenon with the strongest oxidizing agent, fluorine, are known. Who knows, maybe they will succeed with this daring idea with the help of carborane acid.
The chemical synthesis of carborane acid is, of course, a major achievement of Russian and American scientists. This strong acid is subject to study and, for sure, will find application in the creation of new "outlandish" substances.
There are many acids that, even in the smallest amount, are dangerous to humans. Many people believe that sulfuric acid is the most dangerous, but this is absolutely not the case. Carboranoic acid is considered the strongest, which can only be stored in special vessels. It is many times stronger than sulfuric acid and allows you to quickly dissolve metals, glass and other substances that are resistant to other chemicals. But if carborane acid is very rare, and then in laboratory conditions, then another potent substance can be encountered in everyday life. According to many experts, the most poisonous acid is hydrocyanic acid, and it can be found not only in the laboratory, but also in food.
How can you get poisoned
Hydrocyanic acid is very toxic. When it enters the human body, signs of poisoning appear fairly quickly. This substance can enter the body with products that contain it, as well as with those products that have been treated with cyanides.
Most of this poisonous substance is found in almonds. The total amount can be up to 3%. It is enough for a person to eat a small handful of almonds to get poisoned. In addition, such a dangerous substance is found in the seeds of berries and some fruits. Most acids contain:
- peach - up to 2.8%;
- apricot - up to 1.6%;
- plum - up to 0.95%;
- cherry - about 0.8%;
- apple - approximately 0.6%.
In almond seeds and nucleoli of fruits, hydrocyanic acid is not present in its pure form, but in the form of amygdalin glycoside. It is this substance that gives a specific flavor and aroma to nuts. Once in the human body, amygdalin breaks down into three components, one of which is hydrocyanic acid. Bitter almonds are especially rich in this substance, so adults can eat such a product in small quantities, and children should not eat it at all.
Wines made from berries and fruits with stones are of great danger. Wine infused with pitted cherries, plums and apricots can lead to poisoning.
Compotes and jams made from berries along with seeds do not pose a health hazard. When heated already to 80 degrees, hydrocyanic acid decomposes into safe components.
How much acid will cause poisoning
 The amount of food you need to eat to get poisoned can fluctuate significantly. It depends on the age of the person, his body weight, general health and the presence of chronic pathologies. But there are averages that should be followed.
The amount of food you need to eat to get poisoned can fluctuate significantly. It depends on the age of the person, his body weight, general health and the presence of chronic pathologies. But there are averages that should be followed.
The strongest intoxication can be if you eat 30 almonds, more than 50 apricot kernels, more than 70 plum or cherry. You can get poisoned if you eat more than 100 apple seeds.
Under the influence of the most poisonous acid, fatal poisoning can occur. The critical dose of amygdalin is 1 mg per kilogram of human body weight. It is enough to eat 40 grains of bitter almonds or 100 apricot kernels to get fatal poisoning.
Gourmets who are very fond of almond nuts in their unchanged form need to buy a delicacy only in specialized stores. The packaging should contain all information about the manufacturer and the composition of the product. Even sweet almonds can lead to poisoning if eaten without measure.
Bitter almonds are now used only in the manufacture of certain medicines and cosmetic products. Such nuts are practically not eaten.
Symptoms of poisoning
Hydrocyanic acid, once in the bloodstream, binds to red blood cells, while blocking the detachment of oxygen and its further transfer to tissues. Due to this, the amount of oxygen in the blood greatly increases, but it does not enter the organs at all, which leads to hypoxia. The brain is the first to suffer. All functions of this organ are greatly inhibited, and the work of all systems and other important organs in the body is disrupted.
When poisoning with this acid, the following characteristic signs appear:
- skin and all mucous membranes become bright pink;
- severe headache, as well as dizziness, lips go numb and pupils dilate;
- there is an imbalance, a person cannot stand on his feet normally, coordination of movements is disturbed;
- the pulse quickens, as does breathing;
- the victim feels chest pain and shortness of breath;
- nausea and vomiting occurs;
- in the mouth there is a taste of metal and bitterness;
- may have uncontrolled bowel movements.
A characteristic aroma of bitter almonds emanates from the victim, by which it can be determined that the person has been poisoned. If the condition is very severe, then rapid breathing is quickly replaced by a slow pulse. There is paralysis of the respiratory center, and convulsions begin.
If, in case of poisoning with hydrocyanic acid, help is not provided to the victim within 3 minutes, then death will occur.
Urgent care
In case of poisoning with a strong acid - hydrocyanic, you must immediately call an ambulance. Prior to the arrival of doctors, the victim is provided with first aid, which consists of the following measures:

Hydrocyanic acid's antidote is a weak solution of methylene blue. This remedy is usually given by emergency doctors.
After providing first aid, the victim should be removed from all tight clothing and put to bed, raising his head with pillows. If a person has a confused mind, then he is recommended to smell a cotton swab moistened with ammonia. Ammonia, once in the blood, neutralizes the acid.
If a person has no breathing and no pulse, chest compressions and artificial respiration should be performed as soon as possible. Such activities must be carried out in the first few minutes after the cessation of vital processes.
In the conditions of a hospital hospital, the patient is administered anticonvulsants, antidotes and drugs to restore normal blood circulation. In the process of recovery, the patient is shown a complex of vitamins.
After poisoning with hydrocyanic acid, a person should avoid physical and mental stress for some time. At this time, the patient is recommended to drink plenty of fluids, including milk. And you should also walk a lot in the fresh air, adhere to a balanced diet and give up all bad habits.
The rapid development of science allows scientists to make new sensational discoveries in the field of physics, chemistry and other areas. Systematically, the scientific world is shocked by the news about the creation of new substances with unique, never seen before properties. Of course, ordinary people do not always follow such discoveries. Not everyone knows that the strongest acid in the world was created in America in 2005. For many, the strongest chemical of this kind remains sulfuric acid, well studied in school.
Carborane acid is the strongest in the world
In 2005, scientists working at the University of California in the United States managed to create a new acid of unprecedented power. The invented compound is a million times stronger than concentrated sulfuric acid. Scientists at that moment set out to find a new molecule that would be a real discovery in the scientific world, and they managed to achieve a positive result.
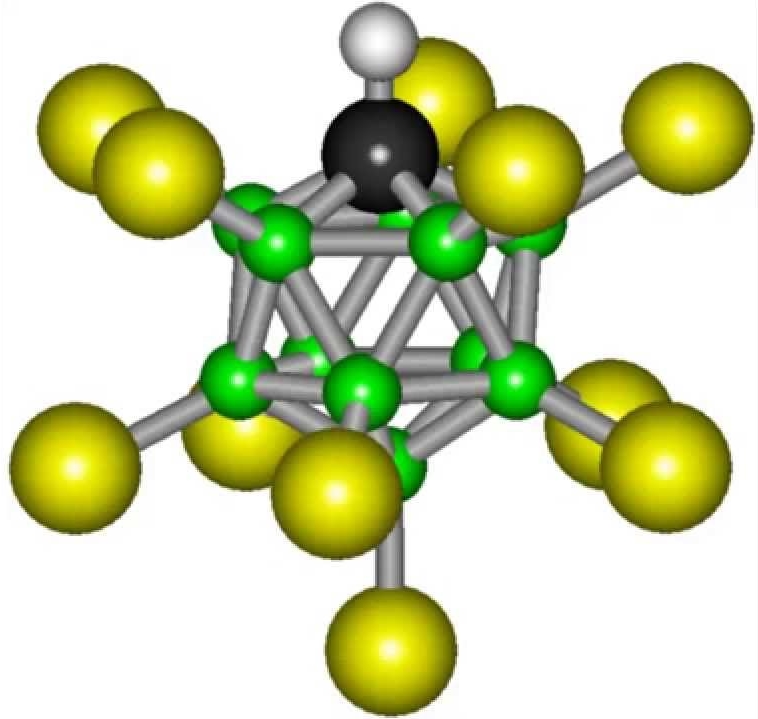
The formula of carborane acid is not complicated: H(CHB11Cl11). But still, it will not work to synthesize such a substance in a conventional laboratory. Carborane acid is more than a trillion times more acidic than ordinary water.
The unique property of the strongest acid
If somewhere the strongest acid in the world is mentioned, the human fantasy draws a substance that dissolves everything in its path. In fact, destructive properties are not at all the main sign of the strength of a chemical. For example, many believed that hydrofluoric acid was the most powerful acid because it dissolves glass. But this is far from the truth. Hydrofluoric acid corrodes glass containers, but can be stored in polyethylene containers.

Recognized as the strongest in the world, carborane acid can be easily stored in glass vessels. The fact is that this chemical is characterized by significant chemical stability. Like other similar compounds, carborane acid, reacting with reagents, donates charged hydrogen atoms. After such a reaction, the composition has a slight negative charge and does not have a destructive effect on the surrounding materials.
Further work with carborane acid
Of course, the creators of carborane acid have become well known in the world scientific community. Moreover, brilliant scientists were awarded many well-deserved awards for their significant contribution to the development of science. The use of the new substance is no longer limited to scientific laboratories: carborane acid is used in industry as a powerful catalyst.

The unique feature of the world's strongest acid is its ability to interact with inert gases. Today, many studies are being carried out, the purpose of which is the possibility of a reaction between xenon and carborane acid. Also, scientists are working tirelessly to study other properties of the most powerful acid.
The best known strong acid
About carboranoic acid is well known to scientists. Simple people Sulfuric acid is often considered the strongest. This is due to the frequent use of the substance in industry. Often it is used by manufacturers of mineral fertilizers to produce superphosphates and ammonium sulfates.
Sulfuric acid is widely used in the metallurgical industry. It is also used to clean metals from oxidation. The production of liquid fuels is not complete without the use of sulfuric acid. It is used to clean the following products:
- lubricating oils;
- kerosene;
- paraffin;
- mineral fats.

But not only industrial use leads many people to believe that sulfuric acid is the most powerful in the world. A similar opinion has developed due to the fact that the substance, falling on the flesh, chars it. This property of sulfuric acid is often used in the filming of crime films.
The strongest organic acid
If we talk about the strongest acid in organic chemistry, then the leadership here belongs to formic acid. The substance was so named because of its discovery in the secretions of ants. Formic acid has a wide range of uses. It is often used in medicine as it has analgesic and irritant properties. Formic acid is present in many ointments that are used to treat bruises, varicose veins, and edema. Medicines with this substance can get rid of acne.

Formic acid is also widely used in the chemical industry. It is also used in agriculture and beekeeping. The substance is also used in food as additive E236.
Despite its prevalence, formic acid can pose a serious threat. Contact with a concentrated substance on the skin causes burns or severe pain. Even inhalation of formic acid vapors can cause damage to the respiratory tract. But the positive property of the substance is that it is quickly excreted from the body without accumulating in it.
More than one generation of chemists argued about which acid is the strongest. V different times this title was given to nitric, sulfuric, hydrochloric acid. Some believed that the compound could not be stronger than hydrofluoric acid. V Lately new compounds with strong acid properties have been obtained. Perhaps it is among them that there is the strongest acid in the world? This article reviews the characteristics of the strongest persistent acids of our time and gives their brief chemical characteristics.
The concept of acid
Chemistry is an exact quantitative science. And the title "The Strongest Acid" should be reasonably attributed to one or another substance. What can be the main indicator that characterizes the strength of any connection?
First, let's remember classic definition acids. Basically, this word is used for complex chemical compounds that consist of hydrogen and an acid residue. The number of hydrogen atoms in the compound depends on the valency of the acid residue. For example, in a molecule of hydrochloric acid there is only one hydrogen atom; and sulfuric acid already owns two H + atoms.

Acid properties
All acids have some chemical properties that can be called common to a given class of chemical compounds.

In all the above properties, another “skill” of any known acid is manifested - this is the ability to donate a hydrogen atom, replacing it with an atom of another chemical substance or a molecule of any compound. It is this ability that characterizes the "strength" of the acid and the degree of its interaction with other chemical elements.
Water and acid
The presence of water significantly reduces the ability of an acid to donate hydrogen atoms. This is due to the fact that hydrogen is able to form its own chemical bonds between acid and water molecules, thus its ability to separate from the base is less than that of undiluted acids.
Superacid
The word "superacid" was introduced into the chemical dictionary in 1927, with the light hand of the famous chemist James Conant.
The standard for the strength of this chemical compound is concentrated sulfuric acid. A chemical or any mixture that exceeds the acidity of concentrated sulfuric acid is called a superacid. The value of a superacid is determined by its ability to impart a positive electrical charge to any base. The corresponding indicator H 2 SO 4 was taken as the basic parameter for determining acidity. Among the strong acids there are substances with rather unusual names and properties.
Known strong acids
The most famous acids from the course of inorganic chemistry are hydroiodic (HI), hydrobromic (HBr), hydrochloric (HCl), sulfuric (H 2 SO 4) and nitric (HNO 3) acids. All of them have a high acidity index and are able to react with most metals and bases. In this series, the strongest acid is a mixture of nitric and hydrochloric acid, called "royal vodka". The formula of the strongest acid in this series is HNO 3 + 3 HCl. This compound is able to dissolve even precious metals such as gold and platinum.

Oddly enough, hydrofluoric acid, which is a hydrogen compound with the strongest halogen - fluorine, did not get into the contenders for the title of "The Strongest Acid in Chemistry". The only feature of this substance is the ability to dissolve glass. Therefore, such acid is stored in polyethylene containers.
Strong organic acids
Contenders for the title "The Strongest Acid in Organic Chemistry" are formic and acetic acids. Formic acid is the strongest in the homologous series of saturated acids. It got its name due to the fact that some of it is contained in the secretions of ants.

Acetic acid is slightly weaker than formic acid, but its distribution spectrum is much wider. It is often found in plant juices and is formed during the oxidation of various organics.
Recent developments in the field of chemistry have made it possible to synthesize a new substance that can compete with traditional organic substances. Trifluoromethanesulfonic acid has an acidity index higher than that of sulfuric acid. At the same time, CF3SO3H is a stable hygroscopic liquid with established physicochemical properties under normal conditions. To date, the title "The Strongest Organic Acid" can be assigned to this compound.
Many may think that the degree of acidity cannot be much higher than that of sulfuric acid. But recently, scientists have synthesized a number of substances whose acidity parameters are several thousand times higher than those of sulfuric acid. Abnormally high values of acidity have compounds obtained by the interaction of protic acids with Lewis acids. In the scientific world they are called: complex protic acids.
Magic acid
Yes. Everything is correct. Magic acid. That's what it's called. Magic acid is a mixture of hydrogen fluoride or fluorosulfonic acid with antimony pentafloride. The chemical formula of this compound is shown in the figure:

This strange name was given to magical acid at a chemists' Christmas party that took place in the early 1960s. One of the members of the research group of J. Olaha showed a funny trick by dissolving a wax candle in this amazing liquid. This is one of the strongest acids of the new generation, but a substance that will surpass it in strength and acidity has already been synthesized.
The strongest acid in the world
Carborane acid - carborane acid, which is by far the most powerful compound in the world. The formula of the strongest acid looks like this: H (CHB11Cl11).
This monster was created in 2005 at the University of California in close cooperation with the Novosibirsk Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences.
The very idea of synthesis arose in the minds of scientists along with the dream of new, hitherto unseen molecules and atoms. The new acid is a million times stronger than sulfuric acid, yet it is completely non-corrosive, and the strongest acid can easily be stored in a glass bottle. True, over time, the glass still dissolves, and with increasing temperature, the rate of such a reaction increases significantly.

This amazing softness is due to the high stability of the new compound. Like all acidic chemicals, carborane acid readily reacts by donating its single proton. In this case, the base of the acid is so stable that the chemical reaction does not proceed further.
Chemical properties of carborane acid
The new acid is an excellent H+ proton donor. This is what determines the strength of this substance. Carborane acid solution contains more hydrogen ions than any other acid in the world. In a chemical reaction, SbF 5 is antimony pentafluoride, it binds fluorine ion. This releases more and more hydrogen atoms. Therefore, carborane acid is the strongest in the world - a suspension of protons in its solution is 2 × 10 19 times greater than that of sulfuric acid.
However, the acid base of this compound is remarkably stable. The molecule of this substance consists of eleven bromine atoms and the same number of chlorine atoms. In space, these particles form a complex, geometrically regular figure, which is called an icosahedron. This arrangement of atoms is the most stable, and this explains the stability of carborane acid.

The meaning of carborane acid
The strongest acid in the world brought its creators well-deserved awards and recognition in the scientific world. Although all the properties of the new substance have not been fully understood, it is already becoming clear that the significance of this discovery goes beyond laboratories and research institutes. Carborane acid can be used as a powerful catalyst in various industrial reactions. In addition, the new acid can interact with the most stubborn chemicals- inert gases. Currently, work is underway to allow the possibility of xenon reacting.
Undoubtedly, the amazing properties of new acids will find their application in various fields of science and technology.
Many people are interested in what is the strongest acid in the world? There has always been a lot of controversy. The title of "the strongest acid" was given to various compounds. V modern chemistry there are new products with more intense properties, but there are organic compounds that are dangerous for any living organism. What acids are in the human body?
Acid is complex chemical compound, which contains hydrogen atoms subject to substitution by metal atoms and an acid residue.
Similar products have different properties and depend on the composition. Acids are in good contact with metals, bases, and are able to change the color of indicators.
According to the presence of oxygen atoms in the compound, they are divided into oxygen and oxygen-free. In the presence of water, the acid "divides" hydrogen atoms to a lesser extent. This is due to the formation of its own hydrogen bond between the molecules of the compound and water, so it does not separate well from the base.
According to the number of hydrogen atoms, acids are divided into monobasic, dibasic and tribasic.
Types of acids (list)
Which connection is considered strong? There is no single answer to such a question. There are superacids that can destroy serious compounds.
Very rare, because it is produced artificially in closed laboratories. There is no exact information about this product, it has been proven that a solution at a concentration of fifty percent is a million times more dangerous than sulfuric acid (also not weak).
Carboranoic acid (the most dangerous)
The compound is considered the stronger of those products that can be stored in specific containers. This corrosive acid is stronger than sulfuric acid. The substance dissolves metals and glass. The compound was created by the joint efforts of scientists from the United States and Russia.
This acid is considered strong due to the easy separation of hydrogen atoms. The remaining ion has a negative charge and high stability, due to which it enters into a repeated reaction. The toxic substance is not a theory, it is used as a catalyst in reactions.
Hydrofluoric acid
Hydrogen fluoride is another strong compound. Available in the form of solutions with different concentrations. The product has no color, heat is released when interacting with water. Toxin destroys glass, metal, does not come into contact with paraffin.
Transported in polyethylene. Hydrofluoric acid is dangerous for humans, causes a narcotic state, circulatory disorders, problems with respiratory system. The compound can evaporate. Vapors also have toxic properties, can irritate mucous membranes and skin. It is rapidly absorbed through the epidermis and causes mutations.
 One of the most common powerful acids. Such a poison is dangerous to humans. When it comes into contact with exposed skin, it causes charring, the appearance of serious wounds that require long-term treatment.
One of the most common powerful acids. Such a poison is dangerous to humans. When it comes into contact with exposed skin, it causes charring, the appearance of serious wounds that require long-term treatment.
Poisoning is dangerous not only when the element enters the body, but also when the vapors are inhaled. sulfuric acid obtained in several ways.
A liquid with a high concentration, when interacting with metal objects, oxidizes them, turns into sulfur dioxide.
Hydrochloric acid
A corrosive acid produced in small quantities in the human stomach. However, a compound obtained by chemical means is dangerous for a living organism. It causes severe burns on contact with the skin, and is very dangerous if it gets into the eyes.
Poisoning is possible with hydrochloric acid vapors; when a container with a substance is opened, a toxic gas is formed that irritates the mucous membranes of the eyes and respiratory organs.
Nitrogen
Refers to substances of the third hazard class. Vapors are harmful to the respiratory tract and lungs, are formed under the influence of elevated temperature. On the skin, the fluid provokes the development of long-healing wounds.
Nitric acid is used in processes, present in fertilizers. However, caution is required when working with it. It does not react with glass, therefore it is stored in it.
Strong organic acids in the world
There are dangerous acids not only of chemical, but also of organic origin. They also carry negative health effects.
Formic acid
Monobasic acid, colorless, soluble in acetone and miscible with water. It is dangerous at high concentrations, when it comes into contact with the skin, it corrodes tissues, leaves severe burns. In a state of gas, it affects the mucous membranes of the eyes and the respiratory tract. If ingested, it provokes serious poisoning with adverse consequences.
Acetic
Dangerous compound used in everyday life. Good contact with water, which reduces its concentration. When ingested, it causes severe burns of internal organs, vapors adversely affect the mucous membranes, irritating them. In high concentrations, it causes severe burns, up to tissue necrosis. Requires immediate hospitalization
hydrocyanic
Dangerous and poisonous substance. Present in the seeds of some berries. If inhaled in small amounts, it causes respiratory failure, headache and other unpleasant symptoms.
When ingested in large quantities, it leads to the rapid death of a person due to paralysis of the respiratory center. If poisoning with hydrocyanic acid salts occurs, the rapid administration of an antidote and delivery to a medical facility is required.
 The title of one of the strongest and most aggressive acids in the world belongs to carborane. This compound came about through experiments by scientists with the goal of creating something sustainable.
The title of one of the strongest and most aggressive acids in the world belongs to carborane. This compound came about through experiments by scientists with the goal of creating something sustainable.
It is stronger than chamois, but does not have the aggressiveness that it has. The composition of the compound includes eleven bromine atoms and the same number of chlorine atoms. In space, the molecule takes the form regular polyhedron- icosahedron.
Due to this arrangement of atoms, the compound is highly stable.
Such an acid is capable of reacting with the most "stubborn" gases - inert ones. Scientists are trying to achieve a reaction with xenon. The strongest acid has brought success to many professors, but research continues.
How much acid can kill a person?
How much poisonous acid does it take to get poisoned or die? Strong acids react immediately, so in some cases a small drop or one breath is enough.
The amount of acid that can provoke poisoning depends on the age of the person, his physical condition, the immune system, the body's ability to resist harmful substances. In children, poisoning develops faster than in adults due to an accelerated metabolism. The exact dosage can be determined by a medical professional.
Acid poisoning symptoms
How does acid poisoning manifest itself? Depending on the type of compound, different symptoms may develop. However, all poisonings are characterized by the presence of the same manifestations.
Signs:
- Pain when swallowing, sore throat, esophagus, stomach. In case of serious poisoning, the development of pain shock is possible.
- Nausea, vomiting. The outgoing masses acquire a black tint due to bleeding in the stomach.
- Rapid heartbeat.
- Severe diarrhea, black stools in the presence of bleeding in the intestines.
- Low pressure.
- Pale skin and mucous membranes, possibly blue upper layer of the epidermis.
- Strong headache.
- Decreased amount of urine.
- Violation of the respiratory process, breathing is frequent, intermittent.
- Loss of consciousness, falling into a coma.
If one of the signs appears, you must immediately call an ambulance team. The life and capacity of the victim depend on the quick reaction of the surrounding people.
Treatment for poisoning
Prior to the arrival of doctors, it is permissible to provide first aid to the victim. In case of poisoning, you can not do without qualified help, but some actions can alleviate the patient's condition.
What to do:
- If gas became the cause of poisoning, then the patient is taken out or taken out to fresh air;
- A person is placed on a horizontal surface, they provide him with complete rest;
- It is forbidden to wash the stomach, this can lead to a second burn of the esophagus;
- Ice is placed on the abdomen, such an action will help stop internal bleeding;
- You can not give a person pills and drink, so as not to provoke negative consequences.
Irritation, a feeling of sand in the eyes, redness are only minor inconveniences with impaired vision. Scientists have proven that vision loss in 92% of cases ends in blindness.
Crystal Eyes is the best remedy for restoring vision at any age.
Further treatment is carried out in the intensive care unit. The doctor examines the patient, selects the appropriate drugs. The accompanying person must tell the doctor about the poisoning that has occurred and the actions taken.
Procedures:
- Gastric lavage using a probe;
- The introduction of medicinal and cleansing solutions using droppers;
- The use of oxygen inhalations;
- Treatment of a state of shock;
All drugs are selected by the doctor depending on the patient's condition and the degree of poisoning. Treatment is continued until the patient is fully recovered.
Consequences and prevention
Acid poisoning is often fatal. With timely treatment, a favorable prognosis is possible, but in many cases a person remains disabled. The action of all acids negatively affects the state of the digestive tract, the brain and nervous system suffer.
It is possible to avoid intoxication by taking care when working with acids. Toxic substances must not be left in places accessible to children and animals. When using toxic compounds, protective clothing is worn, eyes are hidden behind glasses, gloves are present on hands.
The most terrible and dangerous acid is not available to the average layman. However, in laboratories, it is important to be careful when using such substances. If you experience signs of poisoning, you need to immediately contact a medical facility.
Video: list of dangerous poisons






