Viscosity called the ability of a fluid to resist shear forces. This property of a liquid manifests itself only when it moves. Suppose that a certain amount of liquid is enclosed between two flat unbounded parallel plates (Fig. 2.1); the distance between them is P; the speed of movement of the upper plate relative to the lower one - υ.
Experience shows that a layer of liquid immediately adjacent to the wall adheres to it. Hence it follows that the velocity of the fluid adjacent to the lower wall is equal to zero, and to the upper one - υ. Intermediate layers move with a speed gradually increasing from 0 to υ.
Fig. 2.1.
Thus, there is a speed difference between adjacent layers, and mutual sliding of the layers occurs, which leads to the manifestation of the force of internal friction.
To move one plate relative to the other, it is necessary to apply to the moving plate some force G, equal to the drag force of the fluid as a result of internal friction. Newton found that this force is proportional to the speed and, contact surfaces S and is inversely proportional to the distance between the plates n , i.e.
where μ is the coefficient of proportionality, called dynamic viscosity (or dynamic viscosity index).
For a greater clarification of this dependence, it should be attributed to the infinitely small distance between the layers of the liquid, then
where Δ υ is the relative speed of movement of adjacent layers; Δ P - distance between them. Or at the limit
The last expression represents Newton's law for internal friction. The plus or minus sign is taken depending on the sign of the velocity gradient dv / dn.
Since τ = T / S is the shear stress, then Newton's law can be given a more convenient form:
The tangential stress arising in the fluid is proportional to the velocity gradient in the direction perpendicular to the velocity vector and the area along which it acts.
The proportionality coefficient µ characterizes the physical properties of the liquid and is called dynamic viscosity. It follows from Newton's formula that
This expression implies the physical meaning of the coefficient p: if, then µ = τ.
In hydrodynamics, the quantity
called kinematic viscosity (kinematic coefficient of viscosity).
The dynamic viscosity µ decreases with increasing temperature, and increases with increasing pressure. However, the effect of pressure for droplet liquids is negligible. The dynamic viscosity of gases increases with increasing temperature, and changes insignificantly from changes in pressure.
Newton's law for internal friction in liquids differs significantly from the laws of friction in solids. There is friction at rest in solids. In addition, the friction force is proportional to the normal pressure and depends little on the relative speed of movement. In a fluid obeying Newton's law, in the absence of a relative velocity of motion of the layers, there is no friction force. The friction force does not depend on pressure (normal stress), but depends on the relative speed of movement of the layers. Fluids obeying Newton's law are called Newtonian. However, there are fluids that do not obey this law (abnormal fluids). These include various types of emulsions, colloidal solutions, which are heterogeneous bodies consisting of two phases (solid and liquid).
So, clay solutions used in drilling oil wells, some types of oils near their pour point do not obey Newton's law. Experiments have established that in such fluids, motion begins after the shear stresses reach a certain value, called initial shear stress.
For such liquids, a more general dependence for τ (Bingham's formula) is valid:
where τ0 is the initial shear stress; η - structural viscosity.
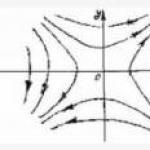
Thus, these fluids at stress τ< τ0 ведут себя как твердые тела и начинают течь лишь при τ ≥ τ0. В дальнейшем градиент скорости пропорционален не т, а разнице τ -τ0.
Graphically, the relationship between and τ is depicted by curve 1 for Newtonian fluids and curve 2 - for anomalous fluids (Fig. 2.2).

Fig. 2.2. Addictiondv / dn from shear stress
When structural fluids move through a pipeline, three modes of their movement are observed: structural, laminar, and turbulent.
Structural. To start moving, a certain initial pressure drop in the pipeline is required Δ R 0, after which the liquid separates from the walls and begins to move as a whole (like a solid).
Laminar. With increasing pressure drop Δ R the velocity of fluid movement will increase and a laminar flow regime will begin to develop near the walls. As the speed increases further, the area of the laminar regime will expand, then the structural regime completely transforms into the laminar regime.
Turbulent. With a further increase in speed, the laminar regime becomes turbulent (see paragraph 6.1).
Dependence of viscosity on temperature and pressure. Viscometers
The viscosity of a dropping liquid depends to a large extent on temperature and to a lesser extent on pressure. The dependence of viscosity on pressure is in most cases neglected. For example, at pressures up to 50-105 Pa, the viscosity changes by no more than 8.5%. An exception is water at a temperature of 25 ° C - its viscosity slightly decreases with increasing pressure. Another feature of water is that its density increases with a decrease in temperature to + 4 ° С, and decreases with a further decrease in temperature (from +4 to 0 ° С). This explains the fact that water freezes from the surface. At a temperature of about 0 ° C, it has the lowest density, and layers of liquid that have such a temperature as the lightest float to the surface, where water freezes if its temperature is less than 0 ° C.
At atmospheric pressure, the viscosity of water depending on temperature is determined by the Poiseuille formula
![]()
Where v - kinematic viscosity; µ - dynamic viscosity; ρ is the density of water at a given temperature; t - water temperature.
The viscosity of a liquid is determined using instruments called viscometers. For liquids more viscous than water, an Engler viscometer is used. This device consists of a container with an opening through which at a temperature of 20 ° C the time for draining distilled water is determined. T 0 and liquid T , the viscosity of which is to be determined. Ratio of quantities T and T 0 is the number of Engler's conditional degrees:
After determining the viscosity of the fluid in conditional Engler degrees, the kinematic viscosity (cm2 / s) is found using the empirical Ubelode formula
![]()
The values of v obtained by this formula are in good agreement with the experimental data.
Let the gas at rest up, perpendicular to the axis x, the plate moves with a speed υ 0, and (υ T is the speed of thermal motion of molecules). The plate carries along the adjacent layer of gas, that layer is adjacent, and so on. All gas is divided, as it were, into thinnest layers, sliding upward the slower the further they are from the plate. Since the layers of gas move at different speeds, friction arises. Let us find out the cause of friction in the gas.

Fig. 3.5
Each gas molecule in the layer takes part in two movements: heat and directional.
Since the direction of the thermal motion changes chaotically, the average thermal velocity vector is zero. With directional movement, the entire set of molecules will drift at a constant speed υ. Thus, the average impulse of an individual molecule of mass m in the layer is determined only by the drift velocity υ:
But since the molecules are involved in thermal motion, they will move from layer to layer. At the same time, they will carry with them an additional impulse, which will be determined by the molecules of the layer to which the molecule has moved. Mixing molecules of different layers leads to equalization of the drift velocities of different layers, which is manifested macroscopically as the action of friction forces between the layers.
Let's go back to fig. 3.5 and consider an elementary site d S perpendicular to axis x... Through this site in time d t flows of molecules pass to the left and to the right:
But these streams carry different impulses: and.
When the momentum is transferred from layer to layer, the momentum of these layers changes. This means that a force equal to the change in momentum acts on each of these layers. This power is nothing but friction force between layers of gas moving at different speeds. Hence the name - internal friction .
Viscosity law was discovered by I. Newton in 1687.
Transportable in time d t impulse is equal to:
From this we obtain the force acting on a unit of surface area separating two adjacent gas layers:
Internal friction in solids can be caused by several different mechanisms, and although they all ultimately lead to the conversion of mechanical energy into heat, these
mechanisms include two different dissipative processes. These two processes are, roughly speaking, analogs of viscous losses and losses by heat conduction during the propagation of sound waves in liquids.
The first type of process depends directly on the inelastic behavior of the body. If the stress-strain curve for a single oscillation cycle has the form of a hysteresis loop, then the area enclosed within this loop represents the mechanical energy that is lost in the form of heat. When a sample is “statically” in a closed stress cycle, a certain amount of energy is dissipated and this loss represents a fraction of the specific scattering caused by the vibration of the sample. As Gemant and Jackson have shown, even when the hysteresis loop is so narrow that it cannot be measured statically, it has a significant effect on the damping of oscillations, since in the experiment on oscillations the sample can perform a large number of closed hysteresis cycles. The energy loss per cycle is constant, so that the specific scatter and therefore the logarithmic decrement is independent of frequency. Gemant and Jackson found that for many materials the logarithmic decrement is indeed constant over a fairly wide frequency range, and concluded that the main cause of internal friction in these cases may simply be associated with the "static" nonlinearity of the stress-strain dependence of the material. Similar results were obtained by Wegel and Walter at high frequencies.
In addition to static hysteresis, many materials exhibit losses due to velocity fluctuations arising from oscillations, and the forces generating these losses can be considered as having a viscous nature. As we have seen, the presence of such forces means that the mechanical behavior depends on the rate of deformation; this effect is noted, in particular, in organic polymers with long molecular chains. The subject of rheology is mainly this kind of time dependence.
It is possible to distinguish between two types of viscous losses in solids, which qualitatively corresponds to the behavior of the Maxwell and Focht models described in the previous paragraphs. So, when the load is kept constant, this can lead to irreversible deformation, as in the Maxwell model, or the deformation can asymptotically tend to some constant value over time and slowly disappear when the load is removed, as it happens in the Focht model. The latter type of viscosity is sometimes called internal viscosity, and the mechanical behavior of such bodies is referred to as retarded elasticity.
The interpretation of the effects of viscosity in solids on a molecular scale is not entirely clear, mainly because the types of microscopic processes that lead to the scattering of mechanical
energy in the form of heat is still largely a matter of conjecture. Tobolskiy, Powell and Ehring and Alfrey investigated viscoelastic behavior using the theory of velocity processes. This approach assumes that each molecule (or each link in the molecular chain in the case of polymers with long molecular chains) undergoes thermal vibrations in the "energy well" formed by its neighbors. As a result of thermal fluctuations, from time to time, energy appears sufficient for the molecule to leave the well, and in the presence of external forces, diffusion takes place, the same in all directions. The diffusion rate depends on the likelihood of the molecule receiving enough energy to leave the well, and therefore on the absolute body temperature. If hydrostatic pressure is applied to the body, the height of the energy well changes, the diffusion rate becomes different, but remains the same in all directions. With uniaxial tension, the height of the well in the direction of the tensile stress becomes lower than in the direction perpendicular to it. Therefore, molecules are more likely to propagate parallel to the tensile stress than in the direction perpendicular to it. This flow leads to the transformation of the elastic energy accumulated by the body into irregular thermal motion, which on a macroscopic scale is perceived as internal friction. Where the molecules move entirely, the flow will be irreversible, and the behavior will be similar to the Maxwell model, while where the molecular links are entangled, the material behaves like the Focht model and exhibits retarded elasticity.
If we make certain assumptions about the shape of the well of potential energy and the nature of the molecular groups that oscillate in it, then it can be shown (Tobolskiy, Powell, Ehring, p. 125) that the theory leads to mechanical behavior of the body, similar to that described by the spring models. shock absorber discussed earlier in this chapter. This interpretation of the issue emphasizes the dependence of viscoelastic properties on temperature; thermodynamic relations can be derived from this dependence. The main inconvenience in applying the theory to real bodies in quantitative terms is due to the fact that the nature of a potential well for bodies is largely a matter of conjecture and that often several different processes can occur simultaneously. Nevertheless, this is still almost the only serious approach to the molecular explanation of the observed effects, and it provides a reliable basis for future development.
Losses occur in homogeneous non-metallic bodies mainly in the same way as described above, and internal friction is associated with the inelastic behavior of the material rather than its macroscopic thermal properties. In metals, however, there are
losses of a thermal nature, which, generally speaking, are more significant, and Zener considered several different thermal mechanisms leading to the dissipation of mechanical energy in the form of heat.
Changes in body volume must be accompanied by changes in temperature; so when a body contracts, its temperature rises, and when it expands, its temperature decreases. For the sake of simplicity, we will consider the banded vibrations of a cantilever plate (tongue). Each time the tongue is bent, the inside heats up and the outside cools, so that a continuous flow of heat is produced back and forth across the bendable tongue. If the motion is very slow, then the heat transfer occurs isothermally and, therefore, is reversible, and therefore no losses should occur at very low vibration frequencies. If the oscillations occur so quickly that heat does not have time to flow across the tongue, then the conditions become adiabatic and still no loss occurs. With bending vibrations, the periods of which are comparable to the time required for heat flow across the tongue, an irreversible transformation of mechanical energy into heat occurs, which is observed in the form of internal friction. Zener showed that for an oscillating reed the specific scattering is given by the expression
And is the adiabatic and isothermal values of the Young's modulus of the material, is the vibration frequency, is the relaxation frequency, which for a tongue of rectangular cross-section has the expression
![]()
here K - thermal conductivity, specific heat at constant pressure, density, tongue thickness in the plane of oscillation.
Bennewitz and Rötger measured internal friction in German silver tongues during lateral vibrations. The results of their experiments are shown in FIG. 29 together with the theoretical curve obtained using equation (5.60). No arbitrary parameters were used to construct this curve, and the agreement between theory and experiment is strikingly good. It is clear that in the frequency range around (about 10 Hz) the thermal conductivity in the tongue is the main cause of internal friction. It is also seen that at frequencies far from the experimental values of internal friction are higher than those predicted by theory, and this indicates that other influences become relatively more important here. Longitudinal stress will be
generate similar effects, since part of the sample is compressed, while the other is stretched, in which case the heat flux is parallel to the direction of propagation. Since the distance between the regions of compression and rarefaction in this case is equal to half the wavelength, the losses caused by this cause will be small at normal frequencies.

FIG. 29. Comparison of the values of internal friction for German silver plates with lateral vibrations, measured by Bennewitz and Rötger and obtained from the theoretical Zener ratios.
The described type of heat loss takes place regardless of whether the body is homogeneous or not. If the material is not homogeneous, there are additional mechanisms that lead to heat loss. Thus, in a polycrystalline material, adjacent grains can have different crystallographic directions with respect to the direction of deformation and, as a result, receive stresses of different magnitudes upon deformation of the sample. Therefore, the temperature will change from crystallite to crystallite, as a result of which the smallest heat fluxes will occur through the grain boundaries. As in the case of losses due to thermal conductivity during cantilever oscillations, there is a lower frequency limit when deformations proceed so slowly that volume changes occur isothermally without any energy loss, and there is also an upper frequency limit when deformations proceed adiabatically, so again no loss occurs. The loss is greatest when the applied frequency falls within the
between these two limits; the value of this frequency depends on the size of the crystal grain and on the thermal conductivity of the medium. Zener derived an expression for the frequency at which this kind of loss is greatest. This equation is similar to (5.61) and has the form
![]()
where a is the average linear grain size.
Randall, Rose, and Zener measured internal friction in brass samples with different grain sizes and found that at the frequencies used, maximum damping was observed when the grain size was very close to that given by equation (5.62). The amount of internal friction caused by these microscopic heat fluxes depends on the type of crystal structure, as well as on the grain size, and increases with increasing elastic anisotropy of individual crystallites. Zener (pp. 89-90) suggested that at very high frequencies the heat flux is almost completely limited to the immediate vicinity of the grain boundary; this leads to a relationship according to which the specific scattering is proportional to the square root of the vibration frequency. This result was confirmed experimentally for brass by Randall, Rose and Zener. At very low frequencies, on the other hand, heat flux occurs throughout the material; hence the relation is obtained according to which the internal friction is proportional to the first power of the frequency. The experimental results of Zener and Randall are in agreement with this conclusion.
There are two other types of heat loss that need to be mentioned. The first is associated with the removal of heat into the surrounding air; the rate of losses for this reason, however, is so low that it affects only at very low vibration frequencies. Another type of loss can arise due to the lack of thermal equilibrium between the normal modes of Debye oscillations; these losses are analogous to the damping of ultrasound in gases caused by the finite time required for the thermal energy to be redistributed between different degrees of freedom of gas molecules. However, in solids, equilibrium between different modes of vibration is established so quickly that the internal friction caused by a similar cause could be expected to be noticeable only at frequencies of the order of 1000 MHz. The theory of the phenomenon described above was considered by Landau and Rumer and later by Gurevich.
For polycrystalline metals, he investigated the internal friction caused by "viscous slip" at the crystal boundaries. He carried out experiments on the damping of torsional vibrations in pure aluminum and showed that the internal friction in this case
can be accurately calculated under the assumption that the metal at the crystal boundaries behaves in a viscous manner.
There are two other processes occurring in crystalline bodies during their deformation, which could lead to internal friction. The first of them is the movement in crystals of regions of disorder, which are called dislocations. The second process is the ordering of the dissolved atoms when a voltage is applied; the latter takes place when there are impurities dissolved in the crystal lattice. The role of dislocations in plastic deformation of crystals was first considered by Oroven, Palanei, and Taylor, and although it seems likely that the motion of these dislocations can often be a significant cause of internal friction, especially at large deformations, the exact mechanism by which elastic energy is dissipated is currently not clear. (see Bradfield). The effect on internal friction of impurities dissolved in the crystal lattice was first considered by Gorskii and later by Snoek. The reason that the presence of such dissolved atoms leads to internal friction is that their equilibrium distribution in a stressed crystal differs from the equilibrium distribution when the crystal is unstressed. When a stress is applied, it takes time to establish a new equilibrium, so that the deformation lags behind the stress. This introduces the relaxation process, which plays an important role for oscillating stresses, the period of which is comparable to the relaxation time. The rate at which equilibrium is established depends very markedly on temperature, so this type of internal friction must be very sensitive to temperature.
A particular case of internal friction has been found in ferromagnetic materials. Becker and Döring gave an exhaustive review of experimental and theoretical studies for materials of this type on the important for applications problem of the magnetostrictive effect in the excitation of ultrasound. It was found that the internal friction in ferromagnetic materials is much higher than in other metals, and it increases with their magnetization; it also rises rapidly with increasing temperature when the Curie point is reached.
The mechanism that attenuates stress waves in solids, but which, strictly speaking, is not internal friction, is scattering. This phenomenon occurs in polycrystalline metals when the wavelength becomes comparable to the grain size; Meson and McSkeemin measured the effect of scattering in aluminum rods and showed that when the wavelength is comparable to the grain size, the attenuation is inversely proportional to the fourth power of the wavelength. This dependence coincides with that given by Rayleigh (volume II, p. 194) for the scattering of sound in gases.
Viscosity is a property of gases, liquids and solids that characterizes their resistance to flow under the influence of external forces. Let us dwell on the viscosity of gases. Due to viscosity, the speed of movement of different layers of gas is equalized, and this happens because molecules, due to chaotic thermal movement, can move from one layer of gas to another. Moving from a rapidly moving layer to a slower one, the molecules transfer their momentum to the latter. And vice versa, the molecules of the layer moving at a lower speed, passing into the moving fast layer, have a braking effect, since they carry with them the impulse of macroscopic motion that is less than the average impulse of the fast layer. In this way, viscosity - this is the phenomenon of momentum transfer of the macroscopic motion of layers of matter.
Fig. 4.31.
Consider the law governing the viscosity phenomenon. To do this, imagine a viscous medium located between two flat parallel plates (Fig. 4.31), moving at different speeds.
Let one of the plates be at rest, while the other moves at a constant speed v, parallel to the plane of the plates (see Fig. 4.31) - the same can be compared to the relative motion of the plates, each with its own non-zero speed. If there is a viscous medium between the plates, then to move the moving plate at a constant speed (while maintaining a constant distance between the plates), it is necessary to apply some constant force directed along the speed F, since the environment resists such movement. It is obvious that tangential forces will act in the medium between its individual layers. Experience shows that strength F which must be applied to the plate in order to maintain its constant speed, is proportional to the speed v plate and its area S and is inversely proportional to the distance between the plates Lx. In the limit at Dx - "Oh, this force
where n is a constant for a given liquid, called coefficient of dynamic viscosity.
This is the force that must be applied in order for two layers of a viscous medium to slide over one another at a constant speed. It is proportional to the contact area S layers and the velocity gradient du / dx perpendicular to the direction of motion of the layers. This statement is Newton's law of internal friction.
To reveal the physical meaning of the viscosity coefficient p, we multiply the left and right sides of equation (4.192) by At. In this case FAt
Ri (du / dx) 5AA On the left is the value FAt(force impulse) equal to Ar(increment of momentum of the body), i.e.
Where Ap - changing the impulse of the flow element by changing the speed of movement.
Dynamic viscosity coefficient p is numerically equal to the momentum of macroscopic motion, which is transferred per unit time through the section of the unit area of the contacting layers (perpendicular to the axis x in fig. 4.31) with a velocity gradient along the same direction equal to unity. In the phenomenon of viscosity, the transferred quantity is the momentum of the macroscopic motion of molecules G (x) = mv (x). Taking into account (4.181) - (4.185), expressions (4.192), (4.193) for viscous friction give:

Per unit of dynamic viscosity in SI the viscosity coefficient of the medium is taken, in which with a velocity gradient equal to one, an impulse of 1 kg m / s is transferred through an area of 1 m 2. Thus, the unit of the viscosity coefficient in SI is kg / (ms). The unit of viscosity in the CGS system (g / (cm s)), which is called poise (Pz) (in honor of the French physicist J. Poiseuille), is widely used. In tables, viscosity is usually expressed in fractions of a centipoise (cP). The ratio between these units: 1 kg / (ms) = 10 Ps.
In addition to the dynamic viscosity coefficient, for the flow characteristic, the kinematic viscosity coefficient v is introduced, which is equal to the ratio of the dynamic viscosity p of the medium to its density p, i.e. v = p / p. In SI, the unit of kinematic viscosity is m 2 / s. In the CGS, v is measured in Stokes (St): 1 St = 1 cm 2 / s.
The dynamic viscosity of liquids is described by an exponential temperature dependence T like p ~ exp (b / t), with the constant characteristic for each liquid B.
Data on the basic laws and quantities in transport phenomena, i.e. on the coefficients of diffusion, thermal conductivity and viscosity are given in table. 4.5. Estimated values of the coefficients in transport phenomena for gases, liquids and solids are given in Table. 4.6.
- Here p is momentum again, p = mv.
Internal friction I
Internal friction
II
Internal friction
in solids, the property of solids to irreversibly convert into heat the mechanical energy imparted to the body in the process of its deformation. V. t. Is associated with two different groups of phenomena - inelasticity and plastic deformation. Inelasticity is a deviation from the elastic properties when a body is deformed under conditions when there are practically no permanent deformations. During deformation with a finite speed, a deviation from thermal equilibrium occurs in the body. For example, when a uniformly heated thin plate is bent, the material of which expands when heated, the stretched fibers will cool, compressed ones will heat up, as a result of which there will be a transverse temperature drop, i.e., elastic deformation will cause a violation of thermal equilibrium. Subsequent equalization of temperature by means of thermal conductivity is a process accompanied by an irreversible transition of a part of elastic energy into thermal energy. This explains the experimentally observed damping of free bending vibrations of the plate - the so-called thermoelastic effect. This process of restoring the disturbed balance is called relaxation (See Relaxation). During elastic deformation of an alloy with a uniform distribution of atoms of various components, a redistribution of atoms in the substance can occur, associated with the difference in their sizes. Restoring the equilibrium distribution of atoms by diffusion (see Diffusion) is also a relaxation process. The manifestations of inelastic, or relaxation, properties, in addition to those mentioned, are elastic aftereffect in pure metals and alloys, elastic hysteresis, etc. The deformation that occurs in an elastic body depends not only on external mechanical forces applied to it, but also on the body temperature, its chemical composition, external magnetic and electric fields (magneto- and electrostriction), grain size, etc. This leads to a variety of relaxation phenomena, each of which makes its own contribution to V. t.If several relaxation processes occur simultaneously in the body, each of which can be characterized by its own relaxation time (see Relaxation) τ i, then the totality of all relaxation times of individual relaxation processes forms the so-called relaxation spectrum of the given material ( fig.
) characterizing the given material under the given conditions; each structural change in the sample changes the relaxation spectrum. The methods used for measuring V. t. Are: study of the damping of free vibrations (longitudinal, transverse, torsional, bending); study of the resonance curve for forced oscillations (see Forced oscillations); relative dissipation of elastic energy for one period of oscillation. The study of solid state physics is a new, rapidly developing area of solid state physics and is a source of important information on the processes occurring in solids, in particular, in pure metals and alloys subjected to various mechanical and thermal treatments. V. t. At plastic deformation. If the forces acting on a solid exceed the elastic limit and plastic flow arises, then we can speak of quasi-viscous resistance to flow (by analogy with a viscous fluid). V.'s mechanism of t. At plastic deformation significantly differs from the V.'s mechanism of t. At inelasticity (see. Plasticity, Creep).
The difference in the mechanisms of energy dissipation also determines the difference in the values of viscosity, which differ by 5-7 orders of magnitude (the viscosity of plastic flow, reaching values of 10 13 -10 8 n· sec / m 2 is always significantly higher than the viscosity calculated from elastic vibrations and equal to 10 7 -
10 8 n· sec / m 2). As the amplitude of elastic vibrations increases, plastic shears begin to play an increasingly important role in the damping of these vibrations, and the viscosity increases, approaching the values of plastic viscosity. Lit .: Novik A.S., Internal friction in metals, in the book: Uspekhi fiziki metallov. Sat. articles, per. from English, part 1, M., 1956; Postnikov VS, Relaxation phenomena in metals and alloys subjected to deformation, "Uspekhi fizicheskikh nauk", 1954, v. 53, v. 1, p. 87; his, Temperature dependence of internal friction of pure metals and alloys, ibid, 1958, v. 66, c. 1, p. 43.
Great Soviet Encyclopedia. - M .: Soviet encyclopedia. 1969-1978 .
See what "Internal friction" is in other dictionaries:
1) the property of solids to irreversibly absorb the mechanical energy received by the body during its deformation. Internal friction manifests itself, for example, in the damping of free vibrations. 2) In liquids and gases, the same as viscosity ... Big Encyclopedic Dictionary
INTERNAL FRICTION, same as viscosity ... Modern encyclopedia
In solids, the property of solids is irreversibly converted into mechanical heat. the energy imparted to the body in the process of its deformation. V. t. Is associated with two decomp. groups of phenomena of inelasticity and plastic. deformation. Inelasticity represents ... ... Physical encyclopedia- 1) the property of solids to irreversibly convert into heat the mechanical energy received by the body during its deformation. Internal friction manifests itself, for example, in the damping of free vibrations. 2) In liquids and gases, the same as viscosity. * * * ... ... encyclopedic Dictionary
Internal friction Internal friction. Conversion of energy into heat under the influence of the vibrational stress of the material. (Source: "Metals and Alloys. Handbook." Edited by YP Solntsev; NPO Professional, NPO Mir and Family; St. Petersburg ... Metallurgical Glossary
Viscosity (internal friction) is a property of solutions that characterizes the resistance to the action of external forces that cause their flow. (See: SP 82 101 98. Preparation and use of building solutions.)